President Donald Trump chooses to lead the Food and Drug Administration (FDA), Professor at Johns Hopkins School of Medicine Dr. Marty Makary, It was confirmed in the Senate on Tuesday.
He consolidated his confirmation just hours after clearing the last program test vote in the evening.
The Senate voted 56-44 to convene Cloture before final confirmation.
Makary, a former Fox News health contributor, joined the Senate Health, Education, Labor and Pension (Help) committee earlier this month and answered various questions about vaccines, chronic diseases, food safety and abortion.
Top Dem uses the same app in the Atlantic scandal to connect with Steele Dossier author

Fox News contributor Dr. Marty Makary officially confirmed to lead the FDA in Trump’s second administration after a vote Tuesday night. (Reuters)
Nominees face scrutiny at an FDA vaccine meeting during his hearing It is said that Postponed at the last minute.
“So if you get confirmed, will you immediately reschedule the FDA Vaccine Advisory Committee meeting to get the opinions of experts?” Senator Patty Murray, D-Wash.asked Macri at the time.
He replied that he “will reevaluate which topics should be called to members of the Advisory Committee [Vaccines and Related Biological Products Advisory Committee] And it may not be necessary to call. ”
When this response wasn’t good enough for Murray, Macari raised the question and told her to face the Biden administration. “Well, you can ask the Biden administration to choose not to convene a committee meeting to boost the booster vaccine booster,” he said.
“Stop them!”: Democrats clash with Trump’s Social Security candidate

Dr. Marty Makary noted that the Biden administration skipped the decision of a key committee meeting when authorizing the vaccine to respond to Patty Murray’s vaccine. (Reuters)
He refers to the Biden administration’s FDA approval in 2021 to provide FDA approval for everyone over the age of 18.
The press release at the time read the press release, “The FDA did not hold a meeting of the Advisory Committee on Vaccine and Related Bioproducts because the agency had previously convened extensive discussions about the use of booster doses of COVID-19 vaccines and reviewed the FDA’s review in EUA requests from Pfizer’s and Moderna, which raised no questions about the FDA’s review.
Can Congress grant compensation to federal courts through major Trump budget procedures?

Signs were seen outside the FDA headquarters in White Oak, Maryland on August 29, 2020. (Reuters/Andrew Kelly/Archive Photo)
Committee Member Dr. Paul Offit“We are asked to approve a third dose of the vaccine for people aged 16 and older without any clear evidence whether the third dose of the young is valuable if compared to the young.”
Chuck Schumer faces “uphill battle” in a situation of suspicion from the leaders: “When, not
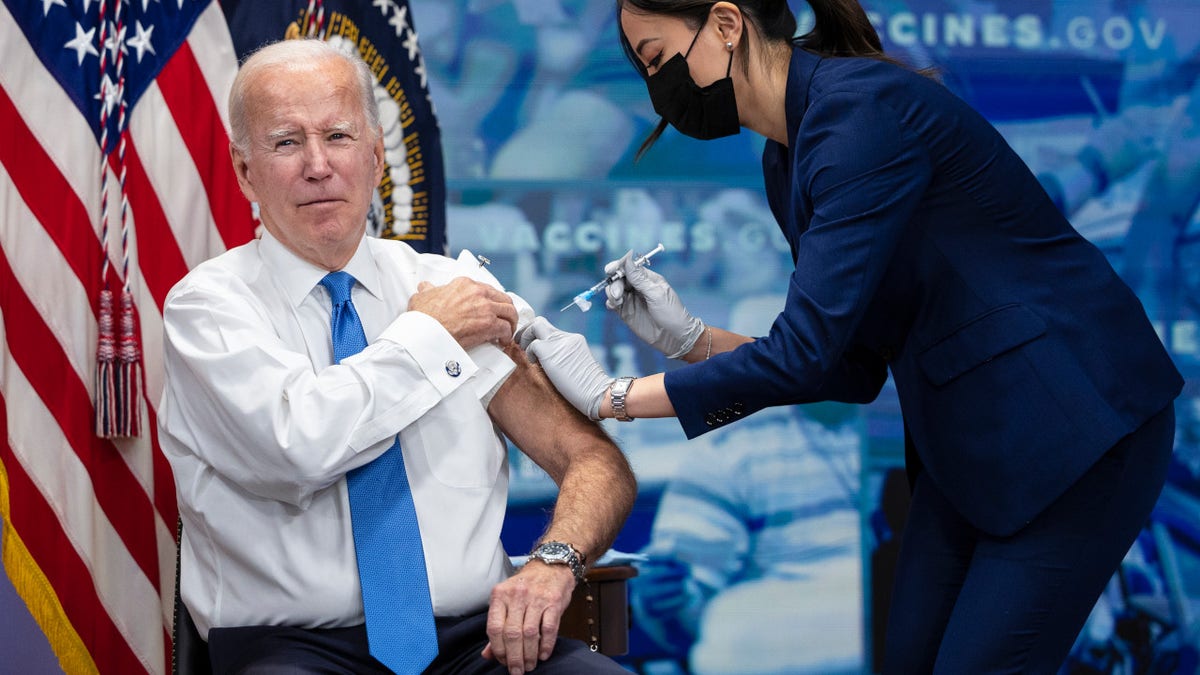
President Joe Biden filmed a Covid-19-19 vaccine booster in the Southern Court Auditorium at the White House in Washington on October 25, 2022. (Tom Brenner via Getty Images in The Washington Post)
Makary has long been a criticism of the government he will now lead. He wrote 2021 opinion articles To “fresh leadership in the FDA can transform the culture of the institution and promote scientific progress rather than hinder it.”
Click here to get the Fox News app
“We now have a generational opportunity in American healthcare,” he said at the hearing. “The focus on healthy foods by President and Secretary Kennedy has inspired grassroots movements in the United States. Childhood obesity is not a problem of willpower, and the rise of early onset Alzheimer’s disease is not a genetic cause. We should do this and we will address food in terms of impacting our health.”


