New extension Prostate cancer drugs New hope can be brought to common patients with this disease.
Novartis, a Swiss-based pharmaceutical company, announced on March 28 that the U.S. Food and Drug Administration (FDA) has expanded its approval of Pluvicto (Lutetium Lu 177 vipivotide Tetraxetan), a targeted radiotherapy (RLT) that is given before chemotherapy.
(RLT is a targeted form of nuclear medicine used by doctors to treat multiple nuclear medicine Cancer Typeaccording to Novartis).
Men who share an unsettling behavior have a 45% increase in risk of prostate cancer
The drug is suitable for patients with PSMA-positive metastatic castration-resistant prostate cancer (MCRPC) who received a round of androgen receptor pathway inhibitor (ARPIS), a drug used to treat metastatic prostate cancer.

Newly expanded prostate cancer drugs may bring new hope to common patients with the disease. (iStock)
Pluvicto first received FDA approval on March 23, 2022, but this new approval tripled the number of eligible patients Receive medicationAccording to Novartis press release.
The drug is injected into the blood through intravenous veins, which attaches to prostate cancer cells and prevents them from replicating or killing them.
“The earlier signs of Pluvicto can indeed change the paradigm of treatment for patients with MCRPC,” said Michael Morris, director of the prostate cancer zone at Memorial Sloan Kettering Cancer Centre in New York.
“It provides a targeted therapy that can better delay disease progression than a second ARPI. This approval is an important step forward and should open the door to the treatment, which has a clear clinical advantage for patients with MCRPC who progressed on one ARPI and did not receive chemotherapy.”
In clinical trials, Pluvicto “reduced the risk of progression or death by 59%.
According to WebMD, this is a prostate cancer that has spread to other parts of the body and has no response to standard hormone treatment.
It also has high levels of prostate-specific membrane antigen (PSMA), a protein produced by prostate cancer cells.
exist Clinical trialsNovartis reported that Pluvicto “significantly reduces the risk of progression or death by 59%” in patients with MCRPC.
image
“FDA’s expanded approval [lutetium Lu 177 vipivotide tetraxetan] Marking a step forward in the treatment of MCRPC, highlighting the growing impact of precision oncology.
Click here to get the Fox News app
“By obtaining this targeted radiation therapy prior to chemotherapy, we not only expand treatment options, but also redefine the standards of care for PSMA-positive diseases.”
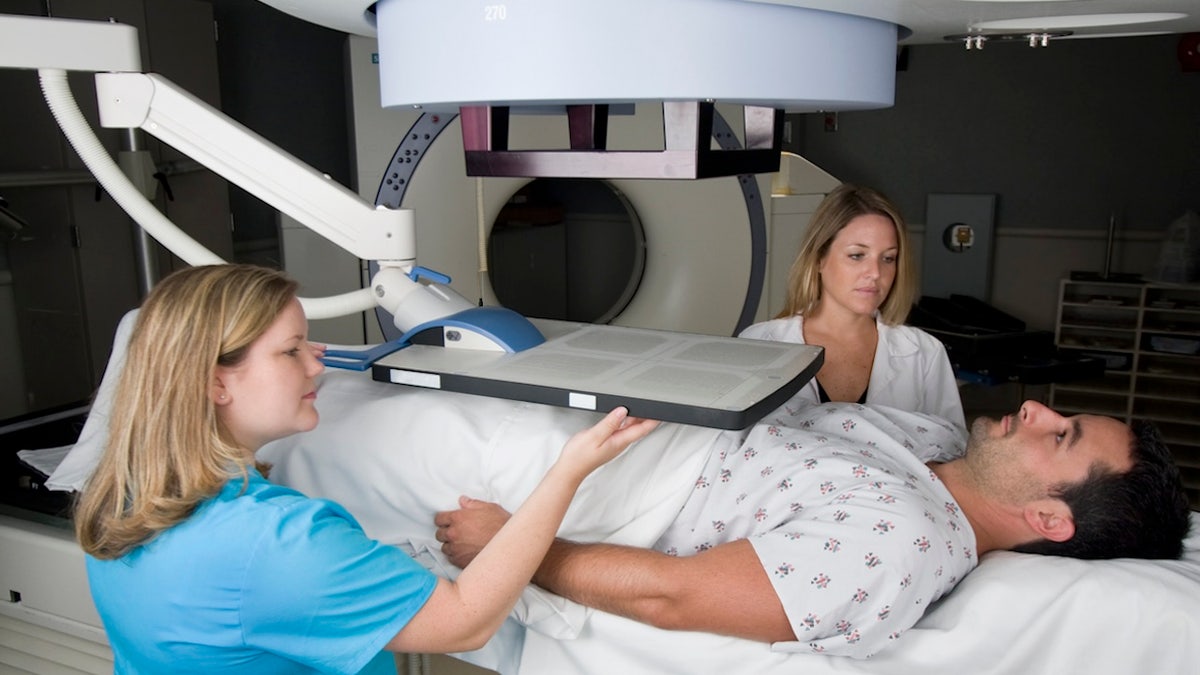
“By obtaining this targeted radiotherapy prior to chemotherapy, we not only expand treatment options, but also redefine the standards of care for PSMA-positive diseases,” one researcher said. (iStock)
Prostate cancer is the second major cause Death between men; MCRPC accounts for the majority of deaths and accounts for 20% of all metastatic prostate cancer cases.
Studies have shown that about 10 to 20% of prostate cancer patients develop MCRPC within five years after initial treatment, with the number of metastatic patients increasing by 4 to 5% per year since 2011.
Click here to sign up for our health newsletter
60% of prostate cancers are diagnosed as male Over 65 years oldaccording to the American Cancer Society. The risk of being diagnosed with metastatic prostate cancer usually occurs between 65 and 74.
Release said that the adverse effects of Pluvicto include dry mouth (61%), fatigue (53%), nausea (32%) and constipation (22%).
Prostate cancer is the second leading cause of death in men. MCRPC accounts for the majority of deaths and accounts for 20% of all metastatic prostate cancer cases. (iStock)
Patients receiving the drug can undergo chemotherapy after taking it.
The company said Novartis is committed to delivering Pluvicto to nearly 600 RLT treatment sites in the United States.
For more health articles, please visit www.foxnews.com/health
Looking to the future, Novartis said it plans to investigate RLT for other types of advanced cancers, including breast, colon, neuroendocrine, lung and Pancreatic cancer.


